Alzheimer’s Disease: Decode the Complex Puzzle
By Dr. Miriam Sonntag from the PAN Academy – our online learning platform where you can learn all about nutrition and health. The purpose of this article is to shed light on the causes of Alzheimer’s disease and how to mitigate them. Learn which dietary choices have the greatest impact. Get actionable dietary strategies to empower your patients to take control of their disease risk and progression.
Alzheimer’s disease, the leading cause of dementia, is a complex disease. Is it caused by a single gene? Does it progress suddenly? Can the risk be modified through lifestyle? Find out what research has to tell.
Alzheimer’s disease vs. dementia
Every 3 seconds someone develops dementia (1). That’s nearly 10 million cases each year. Globally, over 55 million people have dementia, ranking it as the 7th leading cause of death among older individuals (2).
Although often used interchangeably, Alzheimer’s disease and dementia are not the same.
Dementia is an umbrella term for symptoms like memory loss, confusion, and difficulty with daily tasks. In contrast, Alzheimer’s disease is a specific neurodegenerative brain disorder. It’s the leading cause of dementia, accounting for 60-80% of cases. Yet, it’s just one type among several, each characterised by unique brain changes (2).
Image: Dementia – An umbrella term for symptoms affecting cognitive functions and daily activities. Dementia stems from various brain-damaging causes that often coexist.
Brain changes in Alzheimer’s disease
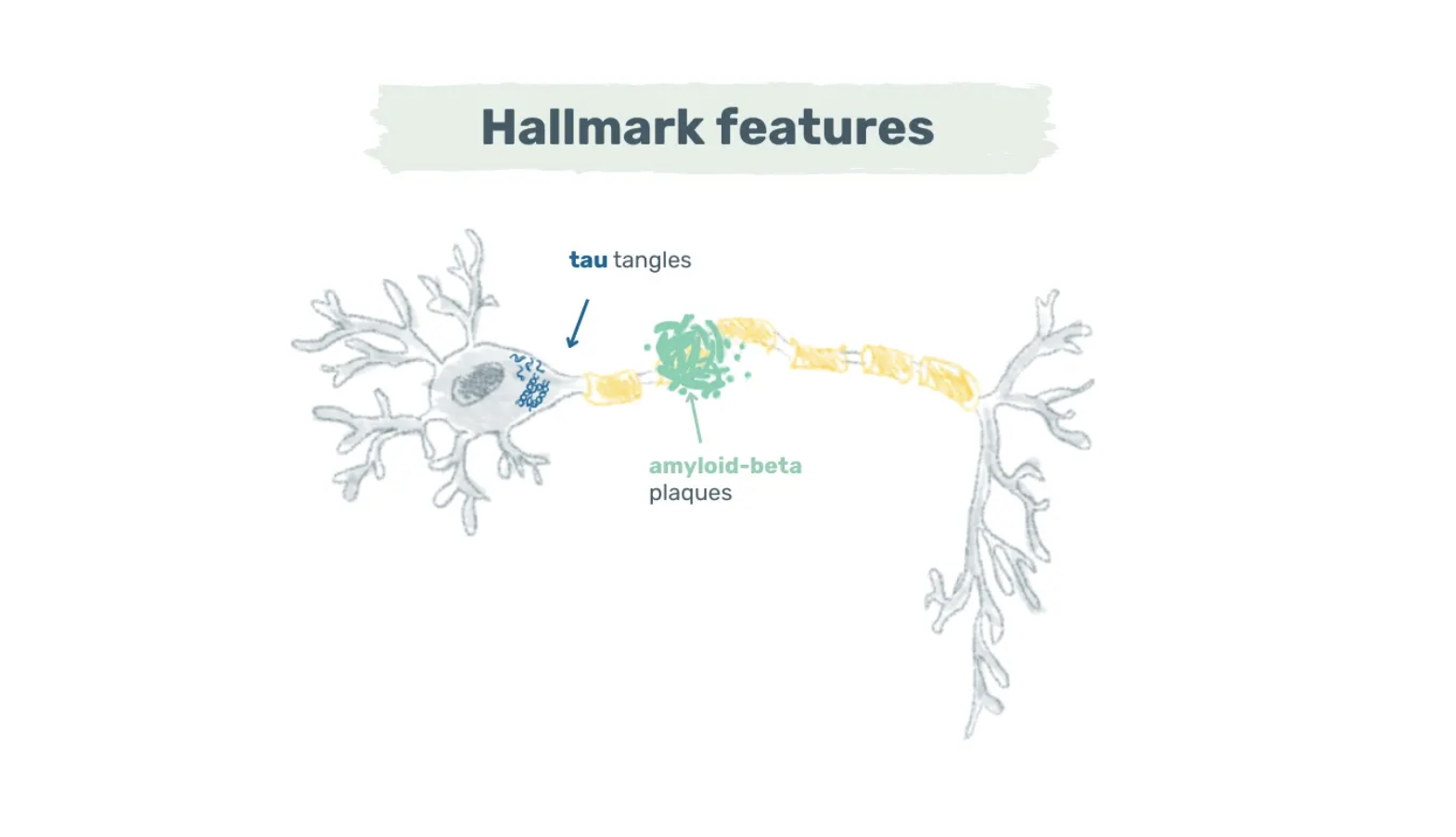
Alzheimer’s disease is a neurodegenerative disease marked by amyloid-beta plaques and tau tangles. While the exact sequence of events is still under study, amyloid-beta plaques typically manifest first. These plaques consist primarily of amyloid-beta, a peptide derived from the amyloid precursor protein expressed in synapses (3).
Image: Hallmark features of Alzheimer’s disease – Amyloid-beta plaques and tau tangles are the two key features of Alzheimer’s disease. Amyloid-beta plaques cluster around neurons, while tau tangles form inside neurons.
In a healthy brain, there’s a balance between the production and clearance of amyloid-beta. In Alzheimer’s disease, however, this balance falters, leading to abnormal levels of amyloid-beta that aggregate into plaques. These plaques cluster around neurons and disrupt synaptic communication (4).
While some plaque build-up is normal during ageing, the point at which the plaques become neurotoxic remains complex and not yet fully understood (4). Once toxic levels are reached, tau inside neurons becomes hyperphosphorylated and aggregates into neurofibrillary tangles.
These tangles impede axonal transport, induce oxidative stress, and eventually lead to neuronal death. Research indicates a strong correlation between the quantity and distribution of tau tangles and cognitive decline (5).
Plaques and tangles are hallmark features of Alzheimer’s disease. Both features can be detected through positron emission tomography scans, plasma or cerebrospinal fluid samples (6). Yet, other changes occur, too.
Elevated levels of amyloid beta and tau activate microglia, the resident macrophages of the central nervous system. Initially, these cells help to clear toxic proteins, pathogens, and damaged neurons. However, when faced with overwhelming amounts of amyloid-beta and tau, microglia may lose their functionality and chronic inflammation may set in.
Chronic inflammation, both a risk factor and a consequence of amyloid-beta plaques, damages neurons and supporting structures. The release of pro-inflammatory cytokines and chemokines not only fuels neuroinflammation but also leads to cell death and brain atrophy (5).
Pathological changes often start in the medial temporal lobe and hippocampus. As neurons die, symptoms such as memory loss, impaired decision-making, or language problems may emerge (7). However, the sequence of affected brain regions, symptom manifestation, and speed of progression may vary from person to person.
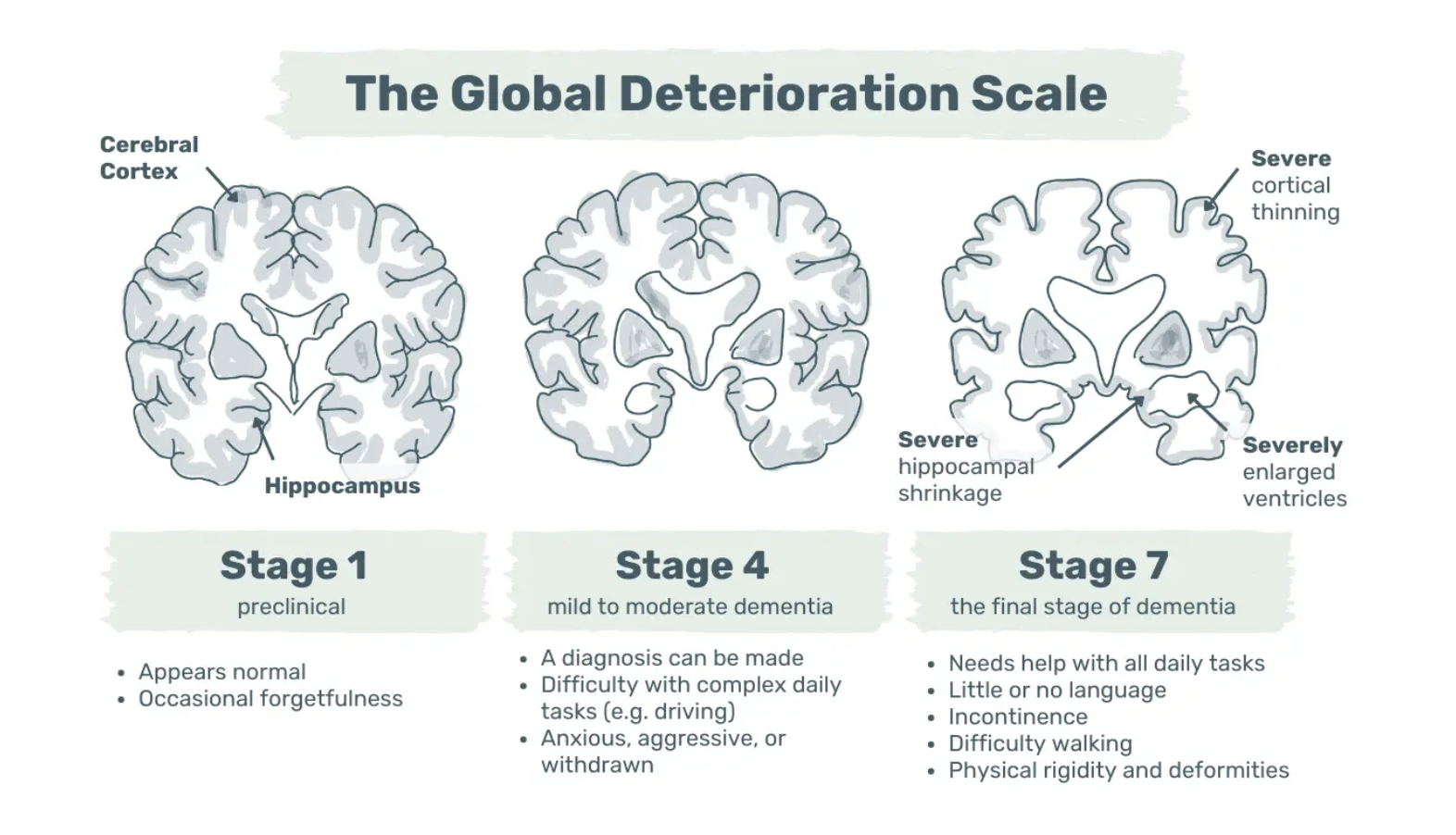
Alzheimer’s dementia progresses through stages, from undetectable brain changes to memory issues and eventual physical disability. In the UK, the NHS and Alzheimer’s Society classify mild, moderate, and severe stages, while the Global Deterioration Scale outlines seven distinct stages (8,9).
Image: The Global Deterioration Scale – Exemplary visualisation of symptoms and brain changes in stages 1, 4, and 7.
Is Alzheimer’s disease genetic?
There are two main types of Alzheimer’s disease: early-onset and late-onset. Early-onset, also known as familial Alzheimer’s disease, results from single-gene disorders in one of the three known risk genes – amyloid-precursor protein, presenilin-1, and presenilin-2 protein.
Inheriting one of these autosomal dominant mutations almost guarantees the development of the disease. Symptoms of cognitive decline often emerge before the age of 65, sometimes as early as 30 (10). However, these mutations are rare, affecting less than 10% of the population (11).
Late-onset Alzheimer’s disease primarily affects individuals aged 65 or older – that’s 90% of those who will develop the disease. In contrast to early-onset, late-onset Alzheimer’s disease is multifactorial. It results from a combination of age, genetics, and lifestyle factors.
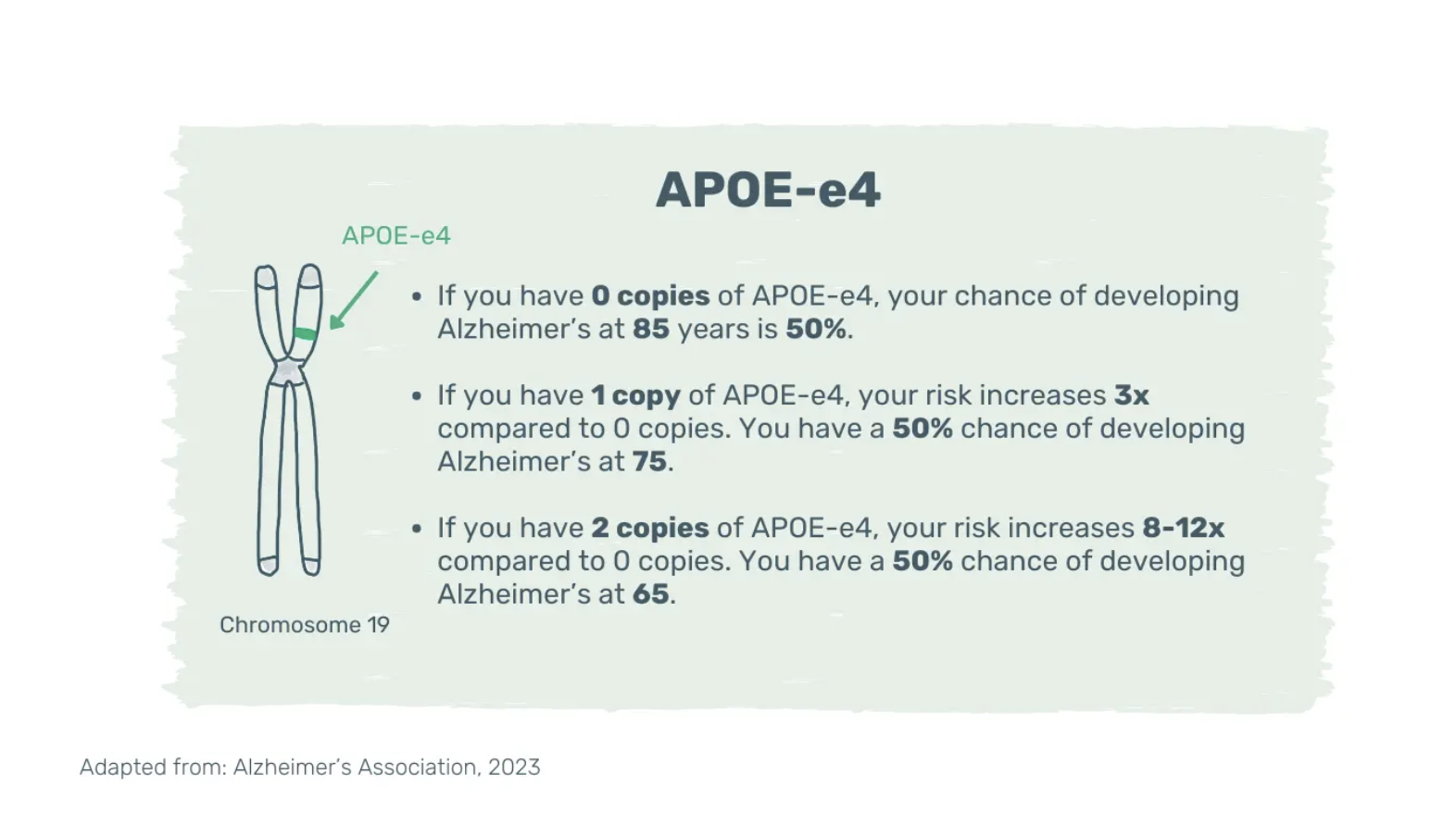
The risk increases with age, a family history of Alzheimer’s disease and the presence of the e4 isoform of the apolipoprotein E (APOE-e4) gene. APOE-e4 is one of several genes associated with late-onset Alzheimer’s disease and its effect is multiplicative.
Having multiple copies of APOE-e4 increases the risk and might lower the age of disease onset (12). However, even two copies of APOE-e4 don’t guarantee Alzheimer’s disease; the disease can develop with or without it and vice versa. Mounting evidence suggests that adopting a healthy lifestyle can counteract the genetic predisposition for dementia (13).
Image: Apolipoprotein E-e4. Genetic variant linked to an increased risk of late-onset Alzheimer’s disease. APOE-e4: E4 isoform of the apolipoprotein E gene.
Is Alzheimer’s disease an inevitable part of ageing?
Age is the strongest known, non-modifiable risk factor for dementia. The risk of developing Alzheimer’s disease doubles every five years after turning 65 and reaches nearly 40% after the age of 85 (14).
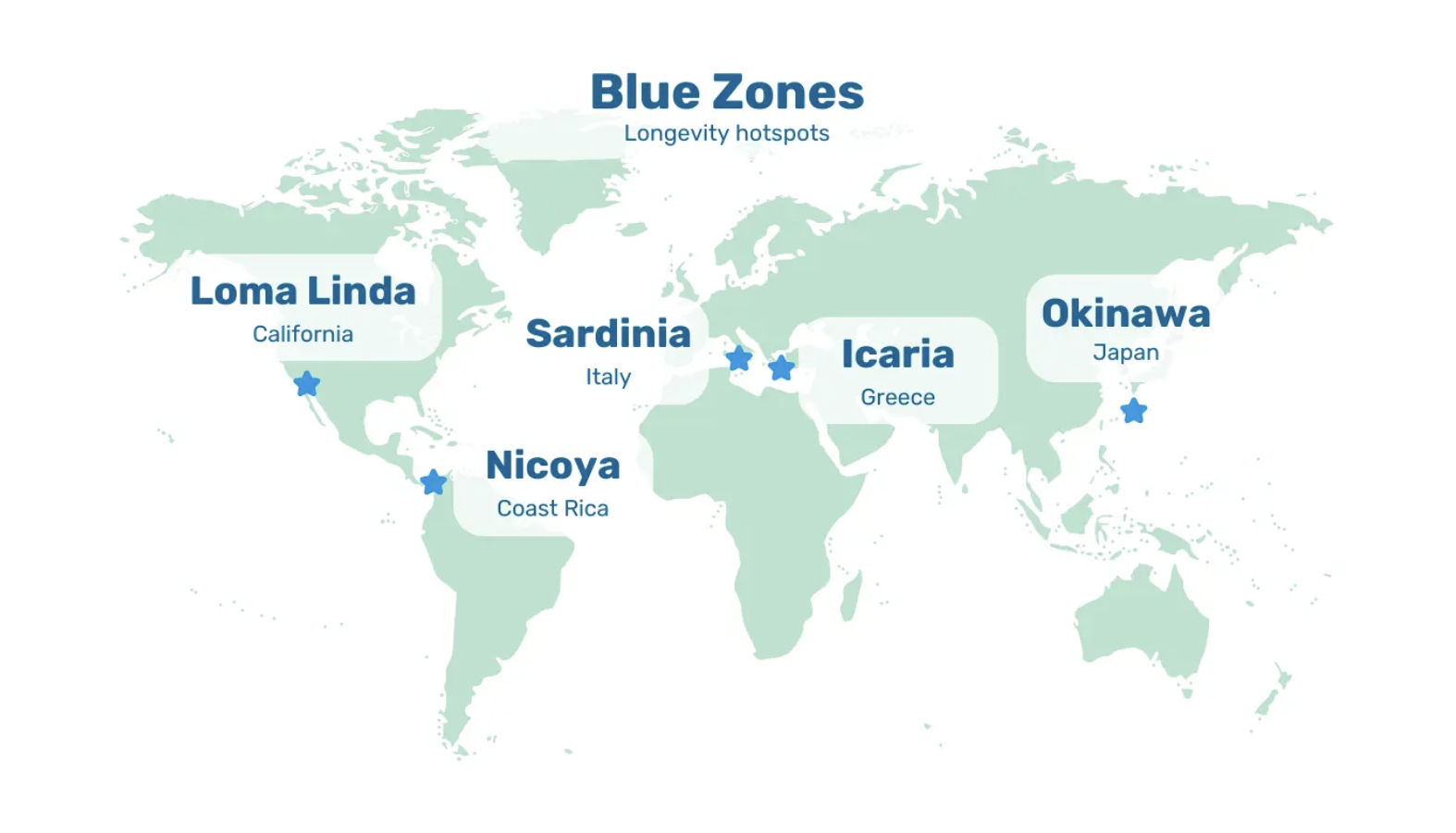
However, even though prevalence increases with age, dementia isn’t inevitable. Take the residents of the Blue Zones, for instance. They not only live a healthy and active life beyond 100 but are less likely to develop cognitive decline or dementia in old age.
Image: Blue Zones. Five geographic areas around the world where inhabitants live measurably longer, healthier and physically active lives beyond 100 years.
Although the Blue Zones are spread across different continents, they share similar lifestyle characteristics. For example, inhabitants of the Blue Zones naturally move throughout the day, maintain strong and close family connections, and eat a predominantly whole food, plant-based diet.
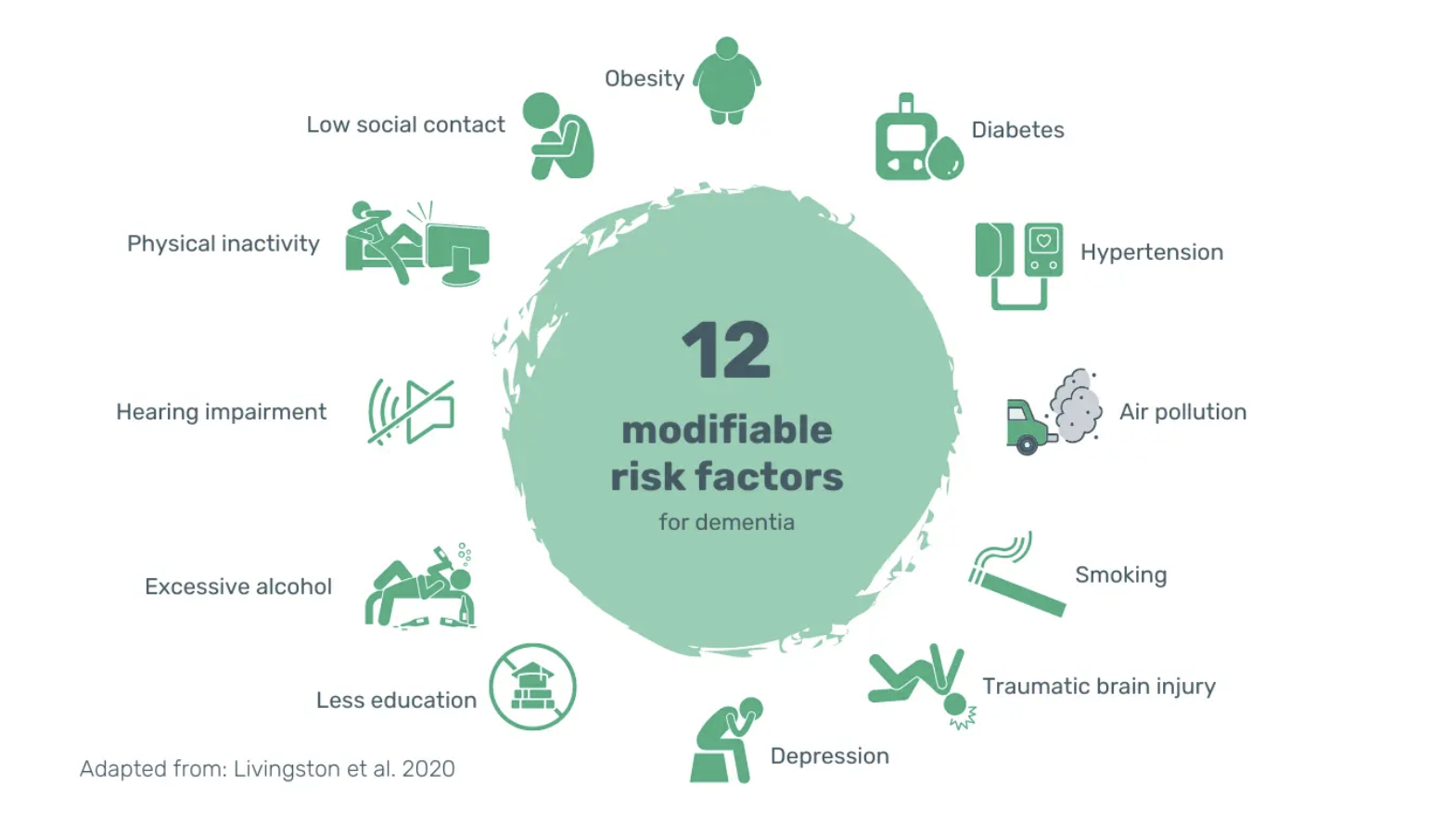
These observations align with the findings in the 2020 Lancet report on dementia prevention, intervention, and care. They estimate that around 40% of all dementia cases could be prevented or delayed by addressing all of the 12 modifiable risk factors below (15).
Image: 12 modifiable risk factors for developing dementia. According to the 2020 Lancet report, these 12 risk factors account for around 40% of all dementia cases.
Even small steps towards a healthier lifestyle can reduce the risk of developing Alzheimer’s disease. In 2020, a study quantified the impact of 5 pre-defined healthy lifestyle factors on Alzheimer’s risk. The researchers tracked 2,675 older subjects over 18 years and gave them lifestyle scores from 0-5 (16).
Image: 5 Healthy lifestyle factors. Subjects received a final score from 0-5, based on how many of the healthy lifestyle factors they met. The higher the score, the healthier the lifestyle. MIND = Mediterranean-DASH Intervention for Neurodegenerative Delay; DASH = Dietary Approaches to Stop Hypertension.
Here’s what they found (16). Compared to those who had ≤ 1 healthy lifestyle factors:
those with 2-3 had a 37% lower risk of developing Alzheimer’s
those with 4-5 had a 60% lower risk of developing Alzheimer’s
While advanced Alzheimer’s disease can’t be reversed, these findings underpin a huge opportunity. The onset of dementia and Alzheimer’s disease can be prevented or delayed by adopting a healthy lifestyle early and maintaining it throughout life (15).
Image: Quote from the 2020 Lancet report on dementia prevention, intervention and care.
Brain healthy diet
The brain, 2% of the body’s weight, demands 25% of the body’s glucose (17). This massive energy demand makes food by far the greatest lifestyle factor in the fight against Alzheimer’s disease.
Observational studies have consistently shown that eating more unprocessed plants and fewer animal products leads to better cognition. Below are just a few of the studies.
A 2019 multi-decade prospective cohort study tracked 16,948 older subjects. Subjects who best adhered to plant-based dietary patterns including the Mediterranean and DASH diet had an 18-33% lower risk of cognitive decline (18–20).
The Rush Memory and Ageing Project analysed data from 923 patients living in retirement communities. Those who strictly adhered to the MIND diet, a hybrid of the Mediterranean and DASH diet, had a 53% lower risk of developing Alzheimer’s disease. Even more remarkable, those who moderately adhered to the MIND diet also reduced their risk by 35% (21).
Compared to the Mediterranean and DASH diets, the MIND diet further reduces fish, meat and cheese intake to once a week (22). It places greater emphasis on dark leafy greens and berries, both of which have been linked to slower cognitive decline (23, 24).
Polyphenols and carotenoids in dark leafy greens and berries have neuroprotective, anti-inflammatory and antioxidant properties. A cohort study of 960 older adults, aged 58-99, concluded that a daily serving of dark leafy greens can slow cognitive decline with ageing. Subjects with the highest intake, about 1-3 servings, had brains that performed as if they were 11 years younger (23).
Berries are rich in flavonols and anthocyanins – two neuroprotective polyphenols. Flavanols have antioxidant and anti-inflammatory properties. Anthocyanins may boost blood flow to the brain and neuroplasticity in the hippocampus (25, 26). Studies have shown that ≥ 1 weekly serving of berries can delay cognitive ageing and reduce the risk of Alzheimer’s dementia (24, 27, 28).
In addition to neuroprotective polyphenols, plants are packed with fibre. Plant fibre triggers the production of short-chain fatty acids in the gut. Short-chain fatty acids reinforce the blood-brain barrier and protect the brain from degeneration (29). Moreover, they have an anti-depressant effect, curb neuroinflammation and disrupt toxic amyloid-beta aggregation (30).
Studies have shown that patients with Alzheimer’s disease have reduced microbial diversity and lower levels of short-chain fatty acid-producing microbes (31, 32). While dysbiosis correlates with disease progression, the opposite is also true. Restoring a healthy microbiome can prevent or slow the progression of Alzheimer’s disease (29).
The Chicago Health and Ageing Project investigated the effects of dietary fats on Alzheimer’s disease risk. They concluded that high amounts of saturated and trans fats may increase the risk, yet fats derived from plants may be protective against Alzheimer’s disease. Subjects with the highest saturated and trans fat intakes had 2.2 and 2.4 times the risk compared to those with the lowest intake (33). A 2012 study, following 6,000 women over 4 years, found that women with the highest unsaturated fatty acid intakes had brain functions as if they were 6 years younger (34). Nuts, seeds, avocados, and olives are good sources of healthy fats.
Take-home message
Whole plant foods are packed with neuroprotective polyphenols, fibre, and unsaturated fatty acids. Although, there’s no cure for Alzheimer’s disease once it has manifested, lifestyle matters. Every step towards more unprocessed plants and fewer ultra-processed food products as well as fewer animal products can help to prevent or slow symptom progression.
Tips for encouraging healthy eating in your patients
Start with the easiest changes your patient can make, even on their hardest day. For lasting results, it’s best to not start with the favourite food right away – instead, cut something else out first. Remember to always suggest healthy and tasty replacements.
Eat a wholefood, plant-based or, at least plant-predominant, diet
Pick healthy fats: Swap saturated fats with healthy, unsaturated fats
Increase fibre intake: Swap refined grains with whole grains and add legumes
Choose healthy proteins: Swap meat and poultry with legumes and soy products
Enjoy a daily dose of leafy greens: Include salad, kale, spinach or rocket
Stock up on flavanols: Include all types of berries every day
Additional Information:
-
Dr. Miriam Sonntag is the Medical Content Executive of the online learning platform, PAN Academy. Having worked in basic research, she knows how to decipher complex information. Working now at PAN, she scans and pours over scientific papers and books. She breaks down the latest nutritional research into actionable advice for everyday life. She is committed to sharing the bigger picture of why it is good to put more plants on your plate.
-
For more information on this topic, download the Alzheimer’s Patient Factsheet.
For more information on this topic, download the Alzheimer’s Physician Factsheet.
-
Alzheimer’s Disease International, 2021. Dementia statistics. Available from: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics. Accessed 8 Feb 2024
WHO, 2023. Dementia. Available from: https://www.who.int/news-room/fact-sheets/detail/dementiaAccessed 8 Nov 2023
Walker LC, 2020. Aβ plaques. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7745791/
Kok FK et al, 2022. Potential mechanisms underlying resistance to dementia in non-demented individuals with Alzheimer’s disease neuropathology. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9198800/
Ferrari C & Sorbi S, 2021. The complexity of Alzheimer’s disease: an evolving puzzle. Available from: https://journals.physiology.org/doi/full/10.1152/physrev.00015.2020
Jack, 2022. Advances in Alzheimer’s disease research over the past two decades. Available from: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(22)00298-8/fulltext
Calabrò M et al, 2021. The biological pathways of Alzheimer disease: a review. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7815481/
Reisberg B & Franssen E, Clinical stages of Alzheimer’s. Available from: https://www.alzinfo.org/understand-alzheimers/clinical-stages-of-alzheimers. Accessed 21 Dec 2023
Alzheimer’s Society, 2021. The progression, signs and stages of dementia. Available from: https://www.alzheimers.org.uk/about-dementia/symptoms-and-diagnosis/how-dementia-progresses/progression-stages-dementia. Accessed 29 Jan 2024
Alzheimer’s Association, 2023. 2023 Alzheimer’s disease facts and figures. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/alz.13016
Robinson M et al, 2018. Recent progress in Alzheimer’s disease research, part 2: genetics and epidemiology. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6705191/
Liu C-C et al, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3726719/
Kuzma E et al, 2019. Genetic risk, lifestyle and dementia. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1016/j.jalz.2019.06.4649
Kumar A et al, 2023. Alzheimer disease. Available from: http://www.ncbi.nlm.nih.gov/books/NBK499922/
Livingston G et al, 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30367-6/fulltext
Dhana K et al, 2020. Healthy lifestyle and the risk of Alzheimer dementia. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7455318/
Ardanaz CG et al, 2022. Brain metabolic alterations in Alzheimer’s disease. Available from: https://www.mdpi.com/1422-0067/23/7/3785
Wu J et al, 2019. Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6766457/
Widmer RJ et al, 2015. The Mediterranean diet, its components, and cardiovascular disease. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339461/
NIH, 2021. DASH Eating Plan. Available from: https://www.nhlbi.nih.gov/education/dash-eating-plan. Accessed 16 Feb 2024
Morris MC et al, 2015. MIND diet associated with reduced incidence of Alzheimer’s disease. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4532650/
Hosking DE et al, 2019. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Available from: https://pubmed.ncbi.nlm.nih.gov/30826160/
Morris MC et al, 2018. Nutrients and bioactives in green leafy vegetables and cognitive decline. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5772164/
Devore EE et al, 2012. Dietary intakes of berries and flavonoids in relation to cognitive decline. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3582325/
Dodd GF et al, 2019. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Available from: https://content.iospress.com/articles/nutrition-and-healthy-aging/nha180056
Shukitt-Hale B et al, 2015. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Available from: https://doi.org/10.1017/S0007114515003451
Agarwal P et al, 2019. Association of strawberries and anthocyanidin intake with Alzheimer’s dementia risk. Available from: https://www.mdpi.com/2072-6643/11/12/3060
Holland TM et al, 2020. Dietary flavonols and risk of Alzheimer dementia. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc7282875/
Silva YP et al, 2020. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2020.00025
Ho L et al, 2018. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958896/
Vogt NM et al, 2017. Gut microbiome alterations in Alzheimer’s disease. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc5648830/
Verhaar BJH et al, 2021. Gut microbiota composition is related to AD pathology. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc8843078/
Morris MC et al, 2003. Dietary fats and the risk of incident Alzheimer disease. Available from: https://doi.org/10.1001/archneur.60.2.194
Okereke et al., 2012. Dietary fat types and 4-year cognitive change in community-dwelling older women. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3405188/